VeriChip - The Rise and Fall of the Human Implant Microchip
 Promotional Keychain from VeriChip with microchip inside (2002)
Promotional Keychain from VeriChip with microchip inside (2002)
One of the world's most controversial technologies to be developed and offered to the public was the VeriChip, an implantable microchip for humans.
First touted as a life saving device for patients with memory issues like Alzheimer's, it would eventually become a very controversial technology.
In 2002, Applied Digital Solutions and Digital Angel introduced an injectable microchip, called the VeriChip. In 2004, they received excusive FDA approval for the use of the Verichip microchip as an injectable implant in humans.
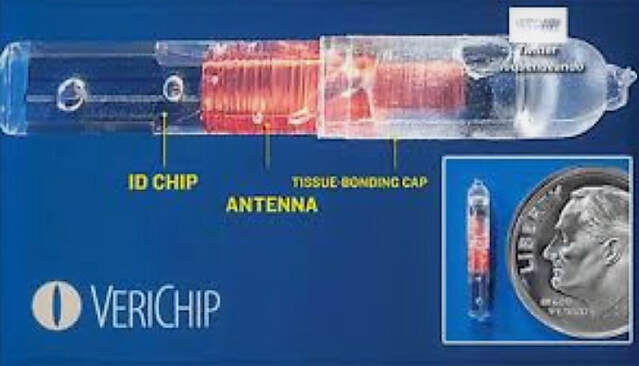
The glass encased VeriChip transponder was designed to hold a 16-digit identification number that could provide vital health information in emergency situations when the patient couldn’t communicate information clearly (such as autism patients).
The injectable microchip RFID transponder was 11 mm by 1 mm in size, about the size of a grain of rice.
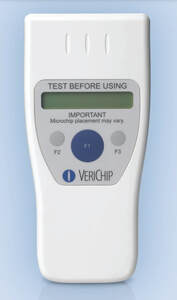
From the VeriMed H2 scanner users manual (2006)
The VeriChip H2 Reader is hand-held Radio Frequency Identification (RFID) reader which activates an implanted VeriMed™ RFID Microtransponder with a low power, low frequency (LF) electromagnetic field. The Reader receives a unique 16-digit ID number from the VeriMed Microtransponder. The ID number is used to provide, with patient consent, physicians and other health professionals access to a secure database that will provide the implanted person’s identity and health information provided by the patient.
The VeriChip H2 Reader is indicated for use as a portable instrument that non-invasively reads the ID number of an implantable VeriMed Microtransponder that is inserted into the arm of the patient. When activated, the VeriMed Microtransponder transmits a unique identification number that will be displayed by the VeriChip H2 Reader and may be used to access the patient’s identity and authorized health information from a secure database.
Historical Timeline of the VeriChip Human Implant Microchip:
- In 1998, Kevin Warwick, a Reading University (London) professor of cybernetics, implanted a chip into his arm as an experiment to see if his computer could track his movements wirelessly around his university building.
- On Sept. 16th 2001, Dr. Richard Seelig, director of medical applications for ADS, injects himself with the first two VeriChip microchip implants to expedite testing of his work, one in the arm and hip.
- In December2001, VeriChip Corp. is created as a wholly owned subsidiary of Applied Digital Solutions.
- In 2002, Journalist Leslie Jacobs, her husband Jeff, and their then-14-year-old son, Derek, became the first of 50 volunteers chosen to be implanted with VeriChip’s controversial RFID chip.
- In 2004, the Baja Beach Club in Barcelona Spain offered their top patrons VeriChip implants to give them special access to VIP areas and to be able to do cashless drink purchases. The program was canceled in 2008.
- In 2004, Mexico’s Attorney General, Rafael Macedo de la Concha, and 160 of his employees were implanted with a VeriChip as a way to secure access to a sensitive records room.
- In 2005, VeriChip Corp. donated implantable chips to the Federal Emergency Management Agency (FEMA) to help track and identify bodies of victims from Hurricane Katrina.
- In 2006, VeriChip Corp. reported that about 200 people worldwide had so far been implanted with their VeriChip microchips. Ethical and privacy concerns about VeriChip and human microchip implanting are starting to being raised.
- In 2006, a study on the authentication and security of the VeriChip microchip are published in the Journal of the American Medical Informatics Association.
- In 2007, a American Medical Association report on RFID tracking on the risks involved with microchip implantation included the issues with migration of the chip under the skin, electromagnetic and electrosurgical interference with devices and defibrillators, and the potential risks associated with certain pharmaceuticals. Also reported, were concerns of risks of privacy, social, and security issues.
- In June 2007 Verichip Corp. announced that 7000 of their microchips have been sold, with around 2000 estimated to have been implanted in humans. Public acceptance of the microchips remains limited.
- From 2007 to 2009, more than 100 patients and caregivers who were clients of Alzheimer’s Community Care in West Palm Beach received VeriChip implants as part of a \research project to provide a safety net for Alzheimer’s patients to help identify them and notify caregivers in case of an emergency.
- In 2009, VeriChip announces they have reduced the length of their implantable microchips to a size of 8mm by 1mm (previous microchips were longer, 11mm x 1mm.)
- In Nov. 2009, VeriChip Corporation and Steel Vault Corporate merge to form PositiveID.
- By 2009, at least nine states have either passed laws or proposed bills to prohibit the enforced implantation of human microchips.
- In July 2010, PositiveID, announces that it would finally discontinued marketing the VeriChip implant microchip due to poor public acceptance.
- In 2011, VeriChip is sold to VeriTeQ Acquisition, owned by PositiveID's CEO.
- In 2012, VeriTeQ merged with Connectyx Technologies, owner of the MedFlash health records system.
- In 2013, "Dangerous Things". a Seattle company founded by Amal Graafstra, started offering their own version of the VeriChip, described as a cybernetic microchip biohacking implant for humans.
- In 2017,Three Square Market (32M), a company that specialized in vending machines and office break room self-service software based in River Falls, Wisconisn, has 50 of their 80 employees voluntarily agreed to be microchipped. the chip, impnated in the hand, allows door access to enter the building, sign into their computer, and pay for snacks — all with a wave of their hand on a sensor. The microchip replaces passwords, ID badges and even credit cards